Learn More About Our Innovative Biotechnology
Press Releases, Publications, & Videos

Publications
ASCO 2024 AVM0703 Poster #7077: Durable complete response and overall survival in patients with heavily pretreated, poor-prognosis non-Hodgkin lymphoma to immuno-activating AVM0703 with few and mild drug-related adverse effects

Press Releases
See a very compelling AVM0703 compassionate use patient story of hope and loving life in Allina Health annual report

Presentation
AVM Biotechnology Provides a Solution to Needed Innovation for Cancer Cures

Press Releases
AVM Biotechnology Selected by the National Cancer Institute to Present at Bio Investor Forum 2023

Press Releases
National Cancer Institute Selects AVM Biotechnology as a 2023-2024 Showcase Company

Press Releases
AVM Biotechnology Announces Twenty-Eight (28) Solid Tumor and Blood Cancer Patients have been Treated with Immunomodulatory AVM0703 through Expanded Access/Compassionate Use Programs

Press Releases
NIH awards AVM Biotechnology SBIR grant to study AVM0703 combined with R-CHOP

Press Releases
AVM Biotechnology Awarded $2 Million National Cancer Institute SBIR Phase II Grant from the National Institutes of Health to Advance AVM0703 in Cancer

Publications
Acute Supra-pharmacologic Weight-based Dexamethasone (AVM0703), 18 mgs/kg Body Weight, Mobilizes Endogenous Bi-specific Natural Killer T-like Cells Independent of Glucocorticoid Receptor Activation

Publications
AVM0703 Mobilization of Endogenous Gamma Delta/Invariant TCR+ Bi-Specific Natural Killer T-like Cells Effective Against Solid Tumors and Blood Cancers

Press Releases
AVM Presents Poster Highlighting the Effects of AVM0703 at the 2022 ASCO Annual Meeting

Press Releases
AVM Biotechnology Founder and CSO to Present Company Information and Clinical Trial Progress at Life Science Innovation Northwest

Press Releases
AVM Awarded $1.6 Million Phase II SBIR Grant to Study AVM0703’s Potential to Reverse Type 1 Diabetes

Publications
AVM0703 Tumor Debulking Enhances Cy/Flu Efficacy

Publications
Very-High Dose Dexamethasone Mobilizes Endogenous Bi-Specific Gamma Delta+ NKT Cells

Presentation
Oncology Tube Profiles Acceleration of Dosing in AVM NHL Clinical Trial

Press Releases
FDA Approves Accelerated Dosing in NHL/Leukemia Clinical Trial

Press Releases
Full Enrollment of First Cohort of Relapsed/Refractory Non-Hodgkin’s Lymphoma Patients dosed with AVM0703
Presentation
Theresa Deisher Speaks at ESWI Influenza Conference

Publications
Pharma’s Almanac Features AVM0703

Press Releases
AVM Biotechnology CEO/CSO Selected to Speak at ESWI Conference in Novel and Outstanding Discoveries Track

Presentation
What is AVM0703? Find Out!

Press Releases
President Receives Dexamethasone Treatment

Press Releases
AVM Receives FDA Permission for Clinical Trials in COVID-19/ARDS

Press Releases
AVM Expands Executive Team, Appoints Janet R. Rea COO

Presentation
Amplifying Scientific Innovation Podcast

Press Releases
AVM’s Dexamethasone Formulation Has Potential For Greater Impact Than Oxford’s

Presentation
Oxford/AVM’s potential COVID-19 Treatment Featured on KOMO News

Press Releases
AVM Awarded SBIR Grant From National Cancer Institute

Press Releases
FDA Approves AVM0703 Clinical Trials in R/R Non-Hodgkins Lymphoma
Presentation
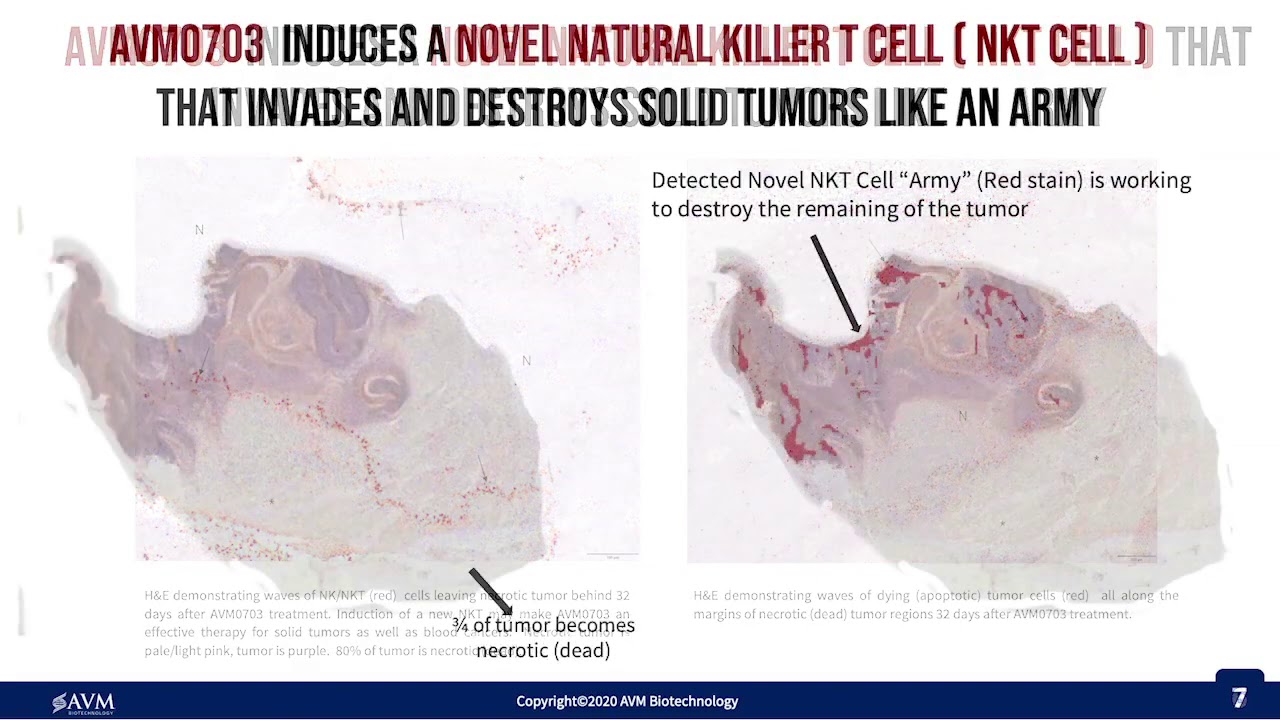
Cavendish Global 2020


